Rev. Bras. Ciênc. Solo.2022;46:e0210146.
Model of inner-sphere adsorption of oxyanions in goethite – Why is phosphate adsorption more significant than that of sulfate?
07/Apr/2022
DOI: 10.36783/18069657rbcs20210146
Graphical Abstract
Highlights
Inner-sphere complexation of PO4 was much more expressive than that of SO4
Oxyanion inner-sphere complexation at pH 5 was more expressive than at pH 9
The negative surface charge density of PO4 defined its greater inner sphere complexation
Inner-sphere complexation depends on the level of ferrol protonation
ABSTRACT
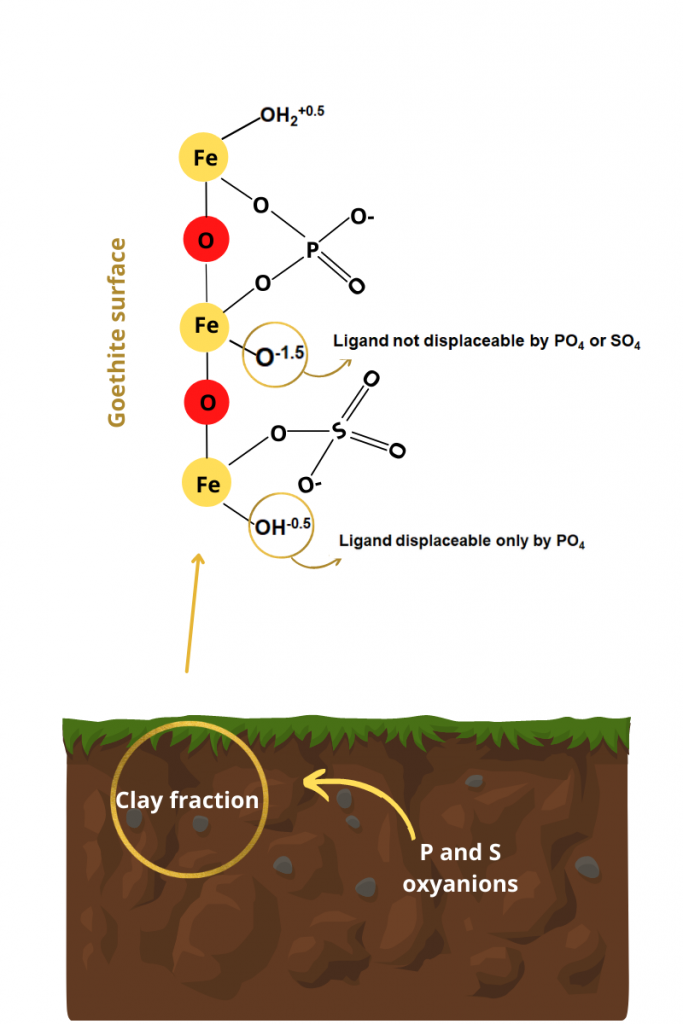
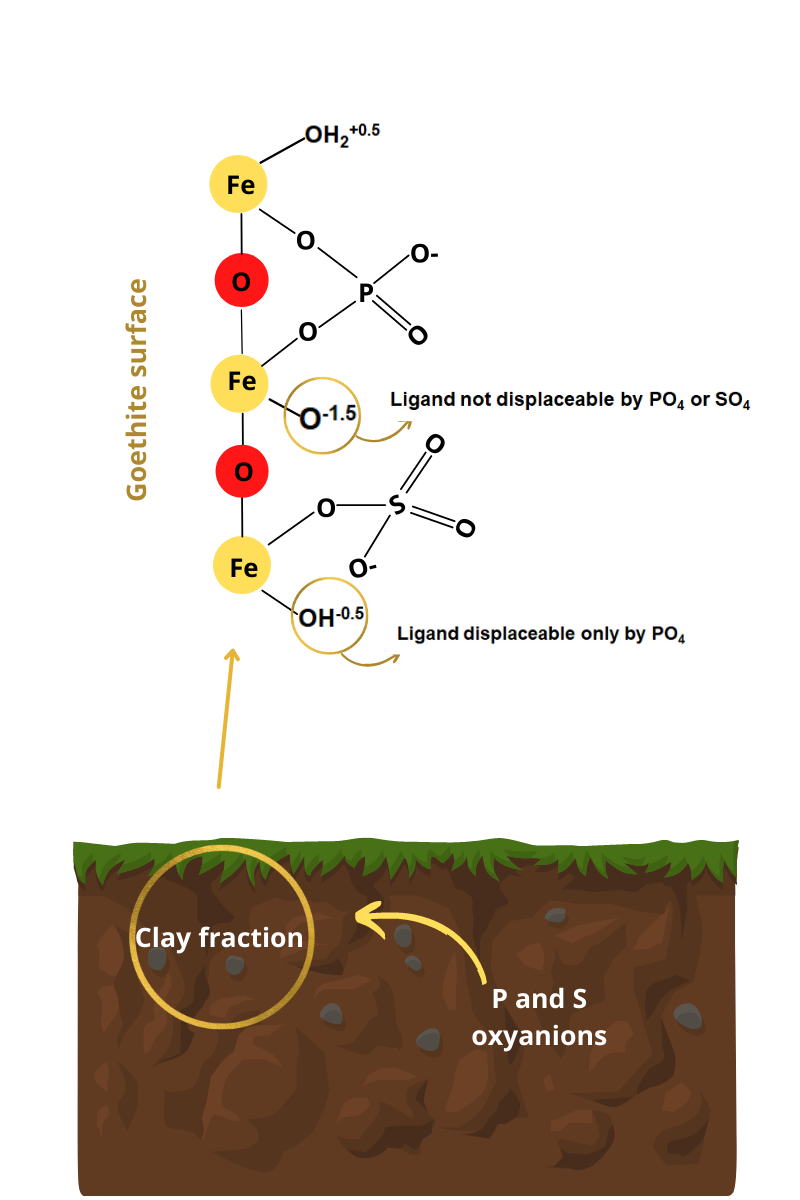
Phosphorus availability in soils is low due to its strong retention by inner-sphere complexation on minerals in the clay fraction with pH-dependent charges, such as goethite. On the other hand, sulfur has greater availability because it is retained mainly by electrostatic attraction. We evaluated the intensities of the inner-sphere complexation of orthophosphate and sulfate (H2PO4-/HPO42- and SO42- – generically treated as PO4 and SO4) under different experimental conditions (pH, goethite purity, and contact times) on synthetic goethite samples to establish the mechanisms and models involved in those bonds. Inner-sphere PO4 and SO4 were extracted using both HNO3 1 mol L-1 and USEPA 3051A methods. Inner-sphere complexation of PO4 and SO4 was highest at pH 5 in relation to pH 9. Attenuated total reflectance/Fourier transform infrared spectroscopy (ATR-FTIR) spectra showed inner-sphere complexation bands of PO4 on goethite in the protonated binuclear bidentate (pH 5) and deprotonated binuclear bidentate (pH 9) forms. Inner-sphere complexation of PO4 was much more expressive than that of SO4. Phosphorus and sulfur oxyanions displace the diprotonated ferrol ligand (-OH2+0.5 in -FeOH2+0.5), while the -OH-0.5 in the -Fe-OH-0.5 group are only displaced by PO4. The -O-1.5 ligand in Fe-O-1.5 group is not displaced by PO4 or SO4. The high surface negative charge density of PO4 defined its higher activation energy for exchanging -OH2+0.5 and -OH-0.5 on the goethite surface in relation to SO4. The proposed model can be used to reduce inner sphere phosphate adsorption in soils and improve P fertilization efficiency for farming.
1,089